
IBS™ Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold System
IRON BIORESORBABLE SCAFFOLD
On May 17, 2024, French time, the world's first product, IBS™ Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold System (hereinafter referred to as " IBS™ Coronary Scaffold"), independently developed by Biotyx Medical ( Shenzhen) Co., Ltd. (hereinafter referred to as "Biotyx Medical") , was selected as the "Best Highlights" of Coronary Artery Devices at the 2024 EuroPCR Conference . The results of the conference selection were announced by Professor Davide Capodanno (Italian cardiologist and editor-in-chief of EuroIntervention magazine) during his closing speech at the main venue of the conference. Only four products were selected as the "Best Highlights" devices of this conference.

Figure 1 Professor Davide Capodanno announced the Best Highlights at the closing ceremony of the conference
At the EuroPCR conference in May 2024, Professor Song Lei of the Fuwai Hospital of the Chinese Academy of Medical Sciences, on behalf of the research team of Academician Gao Runlin, announced the one-year follow-up results of the Phase II clinical trial (IRONMAN-II) of the IBS™ Coronary Scaffold to the world (Figure 3), showing for the first time that the preliminary results of the 12-month IBS™ Coronary Scaffold were comparable to those of the metal everolimus-eluting stent. The IRONMAN-II study was officially launched in March 2022, and it took only 9 months to successfully complete the enrollment of all 518 subjects in 36 centers in China. The 12-month clinical results of the study showed that the TLF (target lesion failure rate) of the IBS™ Coronary Scaffold group was 2.3%, and that of the Xience group was 2.7%. There was no cardiac death or stent thrombosis, and the preliminary safety and effectiveness were comparable to the mainstream drug-eluting metal stents on the market.
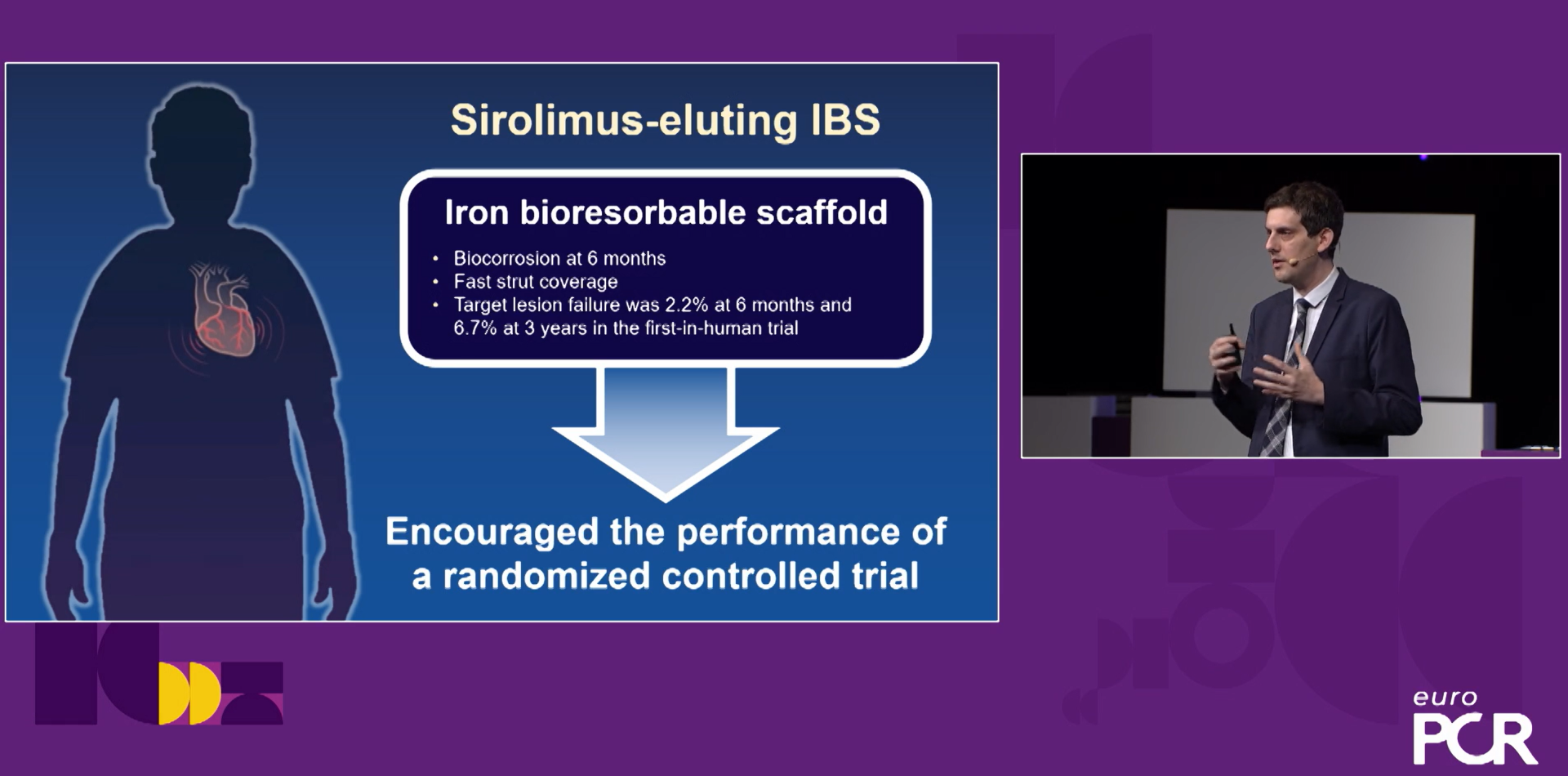
Figure 2 Iron-based scaffold
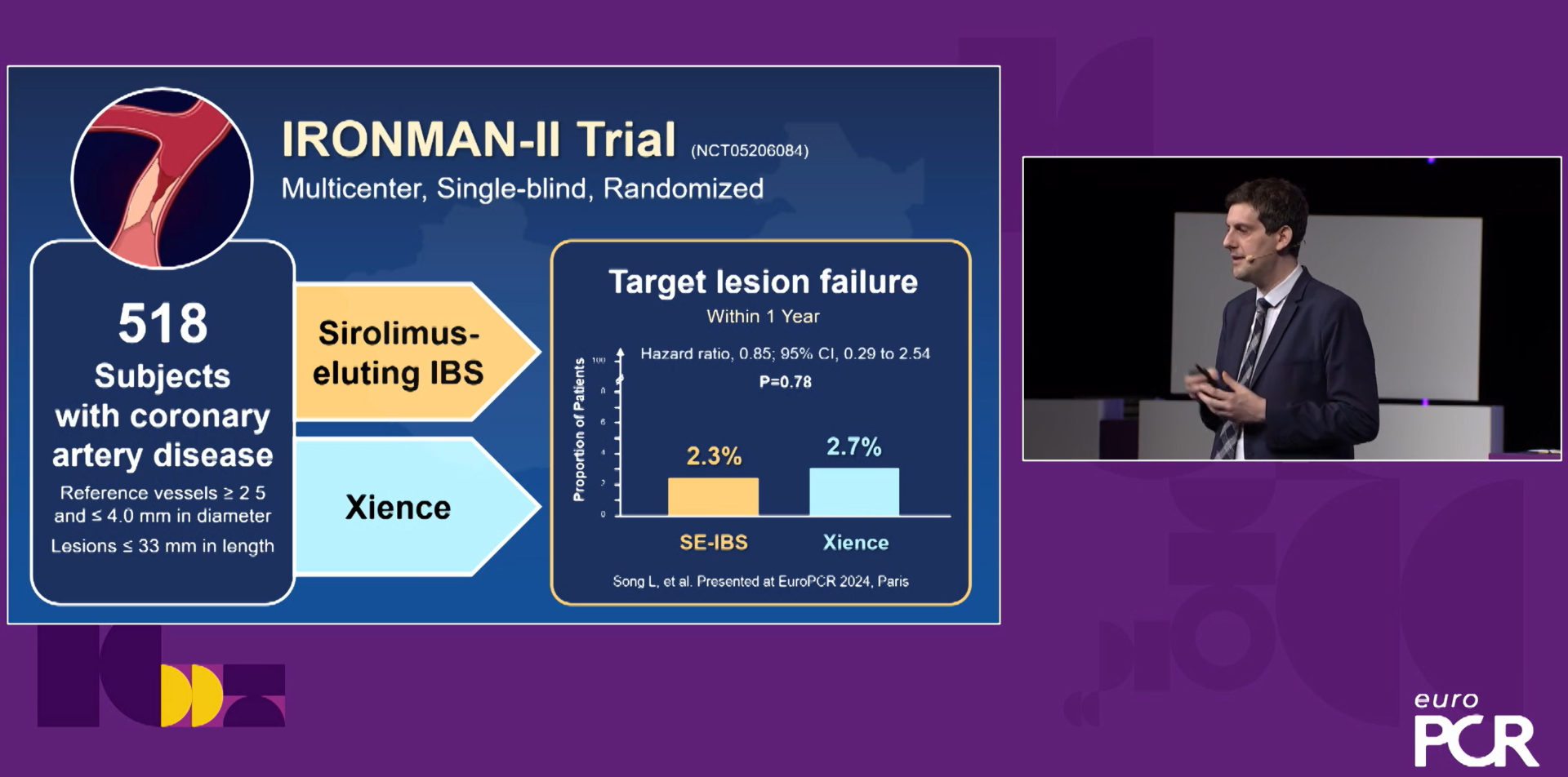
Figure 3 Follow-up data
The IBS™ Coronary Scaffold as one of the four "Best Highlights" devices at the 2024 EuroPCR conference not only represents the unanimous affirmation of the innovation and clinical research of the product by experts and scholars in the field of coronary intervention around the world, but also represents a perfect appearance of China's medical innovation technology at a globally influential professional conference. In his concluding speech at the closing ceremony of the conference, Professor Davide Capodanno reiterated that iron-based absorbable stents are one of the important trends in future coronary artery intervention (PCI) (Figure 4). Fully degradable metal coronary stents made of iron demonstrate great clinical application potential and bright prospects.

Figure 4 Professor Davide Capodanno was concluding speech at the closing ceremony of the conference
IBS™ Coronary Scaffold has submitted an application for EU CE registration, and is expected to become the world's second successfully commercialized iron-based bioresorbable scaffold product after the IBS Angel™ Iron Bioresorbable Scaffold system. With the continuous improvement of subsequent clinical research and evidence-based medicine, the access of this revolutionary innovative product to the global market will be further promoted, bringing unprecedented, safe and effective treatment methods to patients with coronary heart disease around the world in the near future, and will actively promote the treatment of related diseases into the iron-based bioresorbable era!
Article image source: Pcronline
For details of Professor Davide Capodanno’s concluding speech at the closing ceremony of EuroPCR 2024, please visit the following PCR official website:
Scientific highlights, clinical trial updates and key learnings from EuroPCR 2024
