
The latest clinical progress of IBS Titan™ peripheral scaffold
Recently, the innovative IBS Titan™ Sirolimus-Eluting Iron Bioresorbable Peripheral Scaffold System (hereinafter referred to as "IBS Titan™") independently developed by Biotyx Medical (Shenzhen) Co., Ltd. completed the human clinical trial launch meeting of the project at the Azienda Ospedaliero Universitaria Careggi Center in Florence, Italy, and successfully completed the first human clinical implantation in Europe on the same day. This clinical study is led by the Azienda Ospedaliero Universitaria Careggi Center, and Prof. Fanelli serves as the principal investigator (PI). The clinical trial is being carried out in 15 hospitals in multiple European countries including Italy, Germany, Austria, the Netherlands, and Belgium, and plans to enroll 100 patients.

Azienda Ospedaliero Universitaria Careggi Hospital

IBS Titan™ European clinical trial kick-off meeting (the third from the right is Prof. Fanelli, the principal investigator of this clinical trial)
On July 17, local time, Prof. Fanelli and his team successfully implanted a 3.0*28mm IBS Titan™ scaffold into the patient's anterior tibial artery. This was the first surgery in Europe to use IBS Titan™ as the preferred device to treat lower limb arterial ischemia. After the surgery, Prof. Fanelli said that the surgery was very successful . He was sincerely happy that the absorbable drug-eluting peripheral stent could start clinical research in Europe. At the same time, he highly recognized the excellent support, visualization and delivery properties of the IBS Titan™ scaffold and looked forward to the follow-up of this implantation.

Prof. Fanelli's team and Biotyx Medical's team took a group photo after the first patient was enrolled
IBS Titan™ is specially developed for popliteal artery lesions and is used to improve the lumen diameter of the popliteal vessels of patients who need stent treatment. It is the world's first fully degradable peripheral vascular stent with iron as the main material. Its matrix is made of high-strength nitrided iron tubes, and a special coating design is matched to control the degradation rate of the scaffold. At the same time, IBS Titan™ controls drug release through polylactic acid coating (sirolimus), which can effectively inhibit the proliferation and migration of smooth muscle cells.
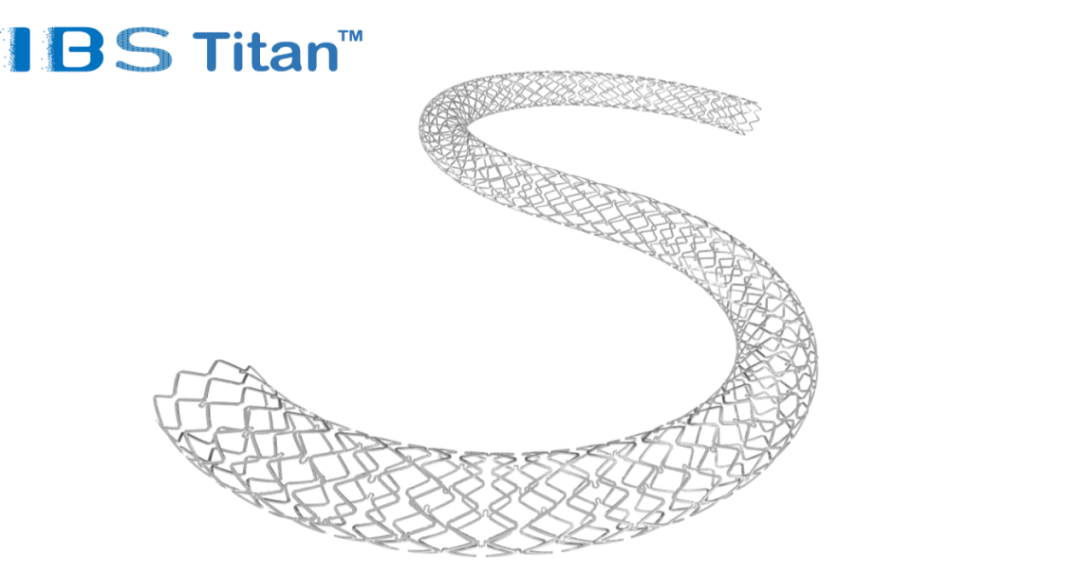
Judging from the relevant data available so far, the mechanical properties and clinical control performance of IBS Titan™ are comparable to those of the mainstream cobalt-chromium alloy permanent stents on the market, and it has a thinner wall thickness and a richer range of specifications than the currently available popliteal peripheral stents. The scaffold can be up to 118mm in length, and can easily enter the diseased blood vessels for vascular patency, and can also be used to treat stenotic lesions within 200mm by stent overlap. Therefore, IBS Titan™ can take into account the treatment of long-segment diffuse calcified lesions, greatly improving the vascular patency rate, while avoiding the disadvantages of low mid- and long-term vascular patency rates of balloon dilatation treatment, the small number of permanent stent specifications, and difficulty in secondary vascular intervention after restenosis.
IBS Titan™ is one of the core products of Biotyx Medical's world-first iron-based bioresorbable material platform. It was approved for "Compassionate Use" by the US FDA in 2021 and successfully implanted. This first clinical enrollment reflects the full recognition of the technical advantages of this innovative product by clinical experts in the core European market. At the same time, IBS Titan™ is carrying out registration clinical-related work in China to obtain more evidence-based medicine to further confirm its safety and effectiveness, and will benefit the majority of patients with lower limb artery disease after the product is successfully launched on the market.
