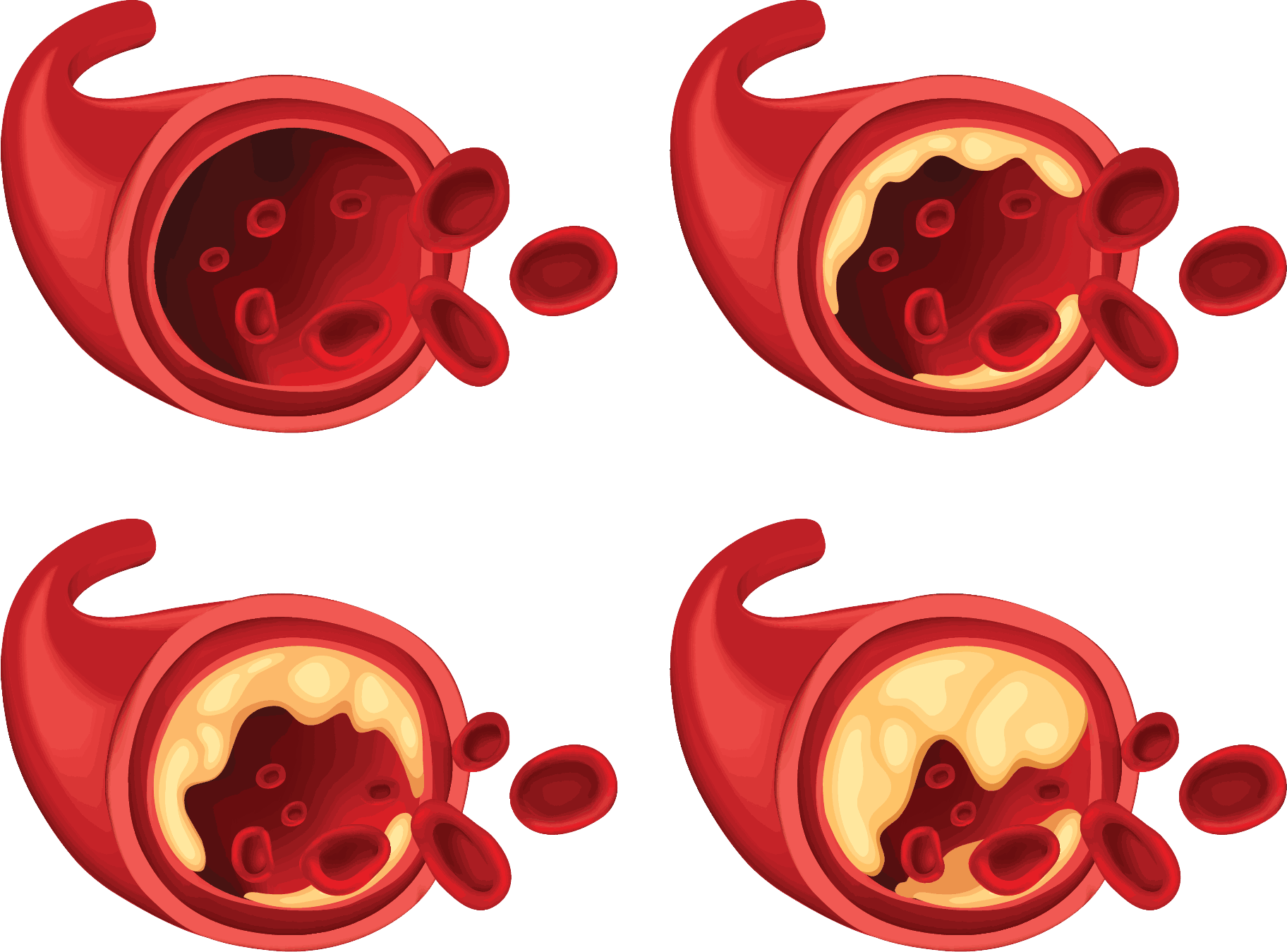
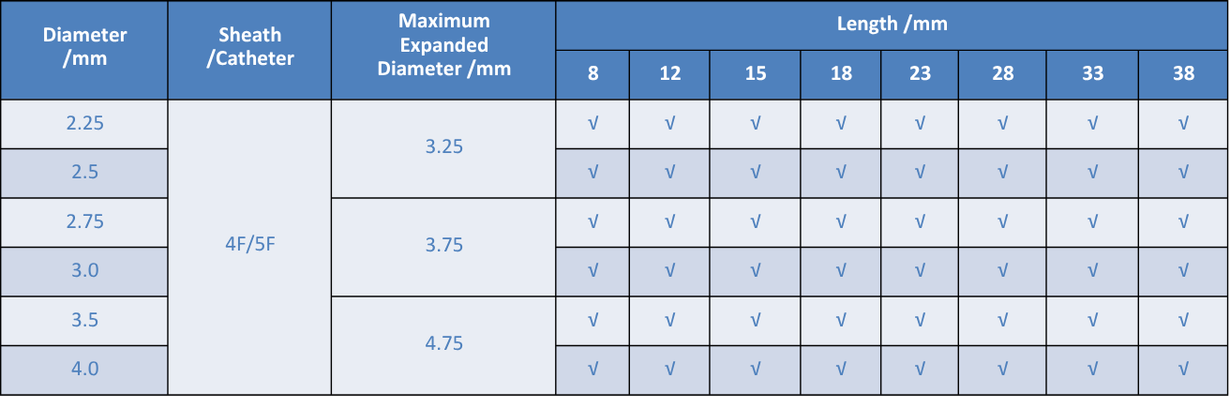
Improving coronary artery luminal diameter in patients with symptomatic heart disease due to de novo native coronary lesions, with reference vessel diameters of ≥ 2.25 mm to ≤ 4.25 mm and lesion lengths ≤ 33mm.
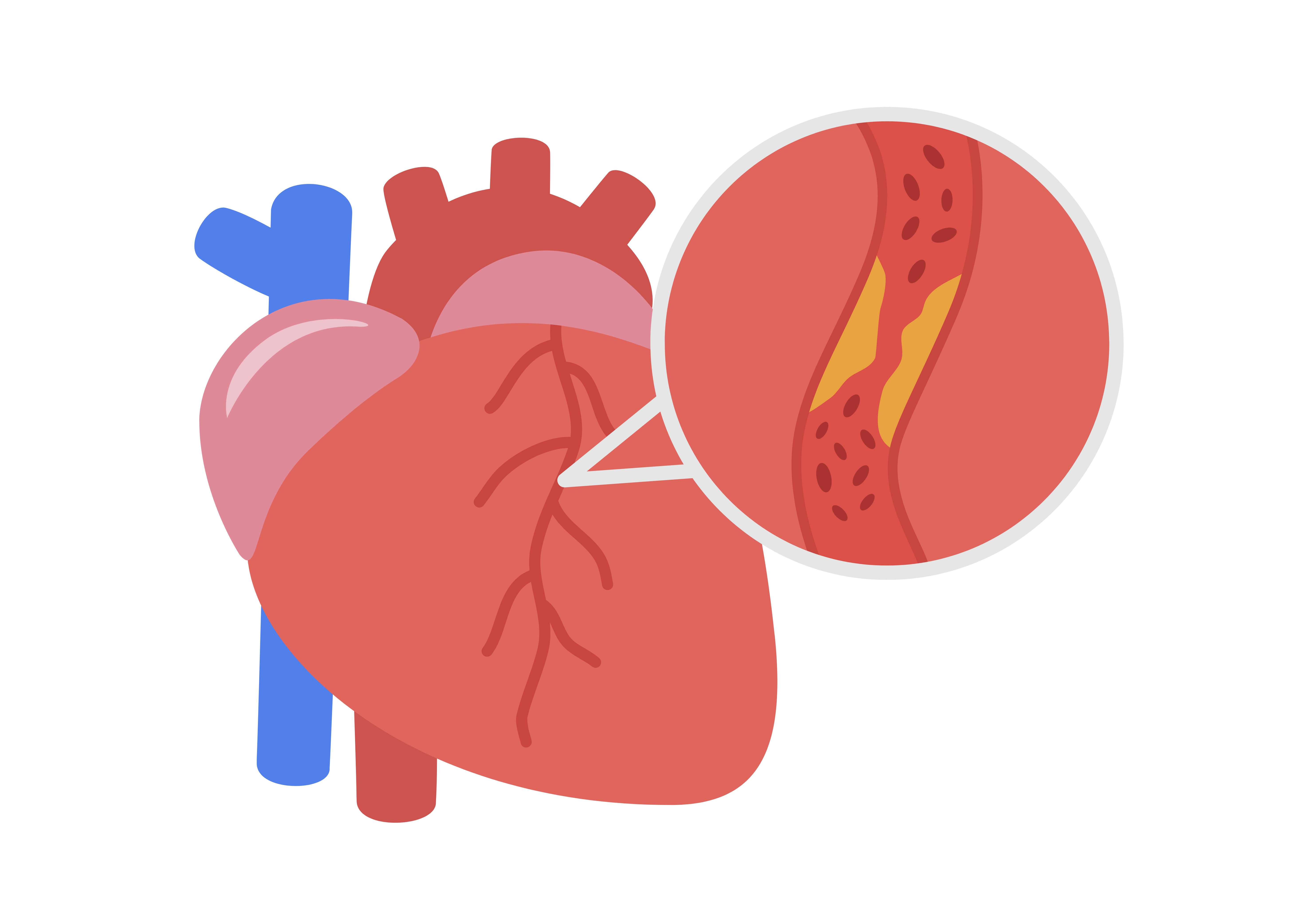



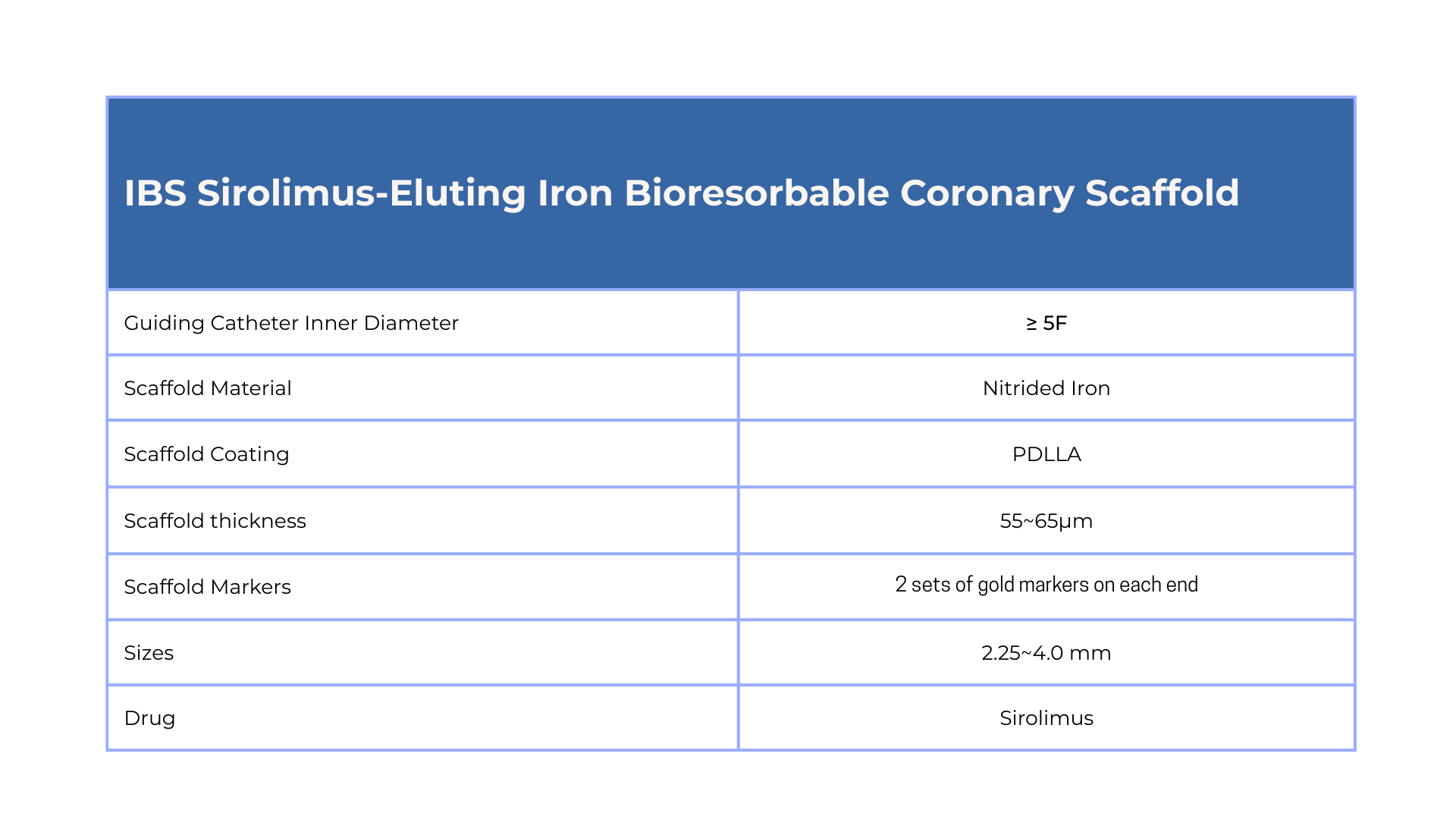
The IBS™ Sirolimus-Eluting lron Bioresorbable Coronary Scaffold comprises a nitrided iron backbone, a zinc buffer layer, and a drug coating layer. As an absorbable scaffold, it finishes degradation in 2-3 years while still maintaining its mechanical properties in the early stage, that can greatly reduce the possibility of vascular collapse.
The degradation products have been verified by multiple institutions multiple times that they will not cause biological toxicity. Furthermore, it is easy to re-intervention after restenosis.

Made from a high-purity nitride iron-tube, with excellent mechanical properties (radial strength), which can provide effective support for blood vessels.
Protect the iron backbone from degradation/corrosion during the early vascular recovery period (3-6 months)
Polylactic acid coating + sirolimus to inhibit VSMC proliferation and reduce the risk of vascular restenosis
Gold-radiopaque markers, enhance scaffold visibility, and facilitate the physicians to position the scaffold and observe during follow-up.
Compared with permanent stents, the lBS scaffold is degradable, and re-intervention is feasible in the same position without causing harm or adding burden to the blood vessels or the human body.
The degradation period is about 2-3 years. lt provides effective support for 6 months after implantation. The scaffold starts to degrade around 3 months after implantation when the blood vessel recovers. And the scaffold will be absorbed by the body 2 years after implantation.
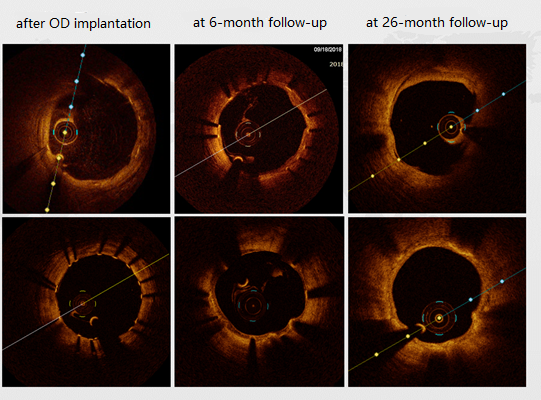
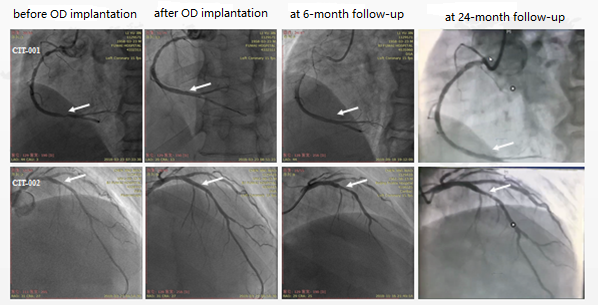
The IBS™ Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold has completed 3 phases of clinical trials in China. Follow-up results show that the clinical results of IBS are comparable to Xience Primes, with no significant difference, proving the safety and effectiveness of iron-based scaffolds once more.
See more details by clicking the bottom below.