The latest clinical progress of BIOTYX MEDICAL IBS coronary scaffold
The world's first product independently developed by Biotyx Medical (Shenzhen) Co., Ltd., the IBS™ Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold System (hereinafter referred to as "IBS™ coronary scaffold"), has successfully completed a one-year follow-up of a randomized controlled study (i.e., "Phase II Clinical Study"). On May 14, 2024, local time in France, Director Lei Song of the Fuwai Hospital of the Chinese Academy of Medical Sciences, on behalf of Academician Runlin Gao and all researchers, announced the one-year follow-up results of Phase II clinical study of IBS™ coronary scaffold for the first time to the world at the 2024 European Congress of Interventional Cardiology ( EuroPCR), a world-renowned and influential cardiac intervention conference, at the Late-Breaking Trials.
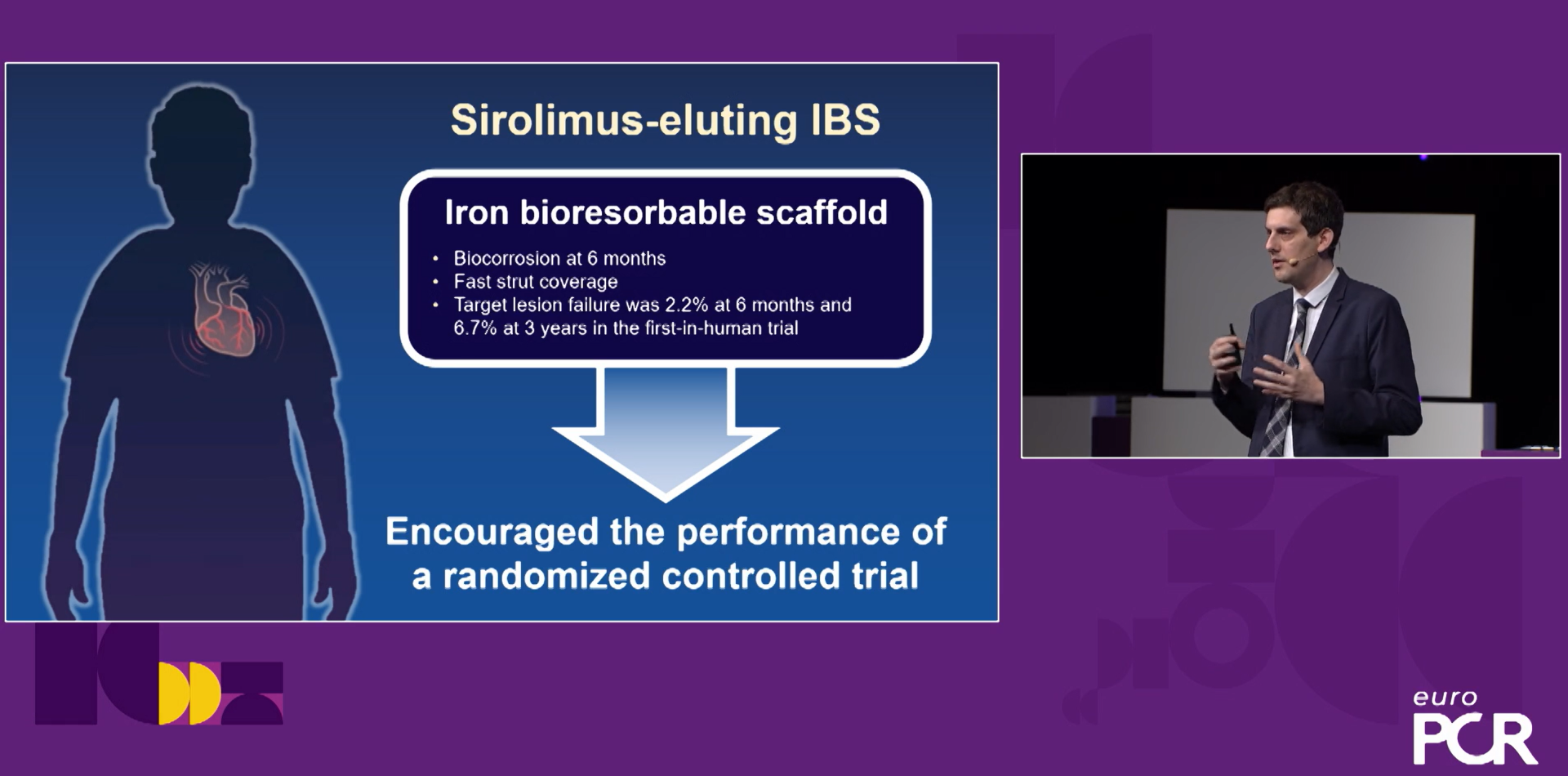
The IBS™ coronary scaffold Phase II clinical study is a prospective, multicenter, single-blind, randomized controlled clinical study. The main endpoint is late lumen loss within the diseased segment two years after coronary stent implantation. The clinical study was officially launched in March 2022. It took only 9 months to successfully complete the enrollment of all 518 subjects in 36 domestic centers and randomly assign them to the trial group (IBS™ coronary scaffold) and 1: 1 . Control group ( Xience™ everolimus drug-eluting coronary stent). One-year clinical follow-up results showed that there was no significant difference in target lesion failure rate (TLF) between the experimental group and the control group (2.3% in the experimental group and 2.7% in the control group, p=0.78); cardiac death (2.7% in the experimental group, p=0.78); The incidence rate of target vessel-related myocardial infarction (0.4% in the experimental group, 1.2% in the control group, p=0.37) and target vessel-related myocardial infarction (0.4% in the experimental group and 1.2% in the control group, p=0.37) was also not significant in the two groups of subjects. There were no device-related thrombotic events in the two groups of subjects, which preliminarily proved that the IBS™ coronary scaffold was non-inferior to the current mainstream drug-eluting metal stents on the market, showing ideal safety and effectiveness.
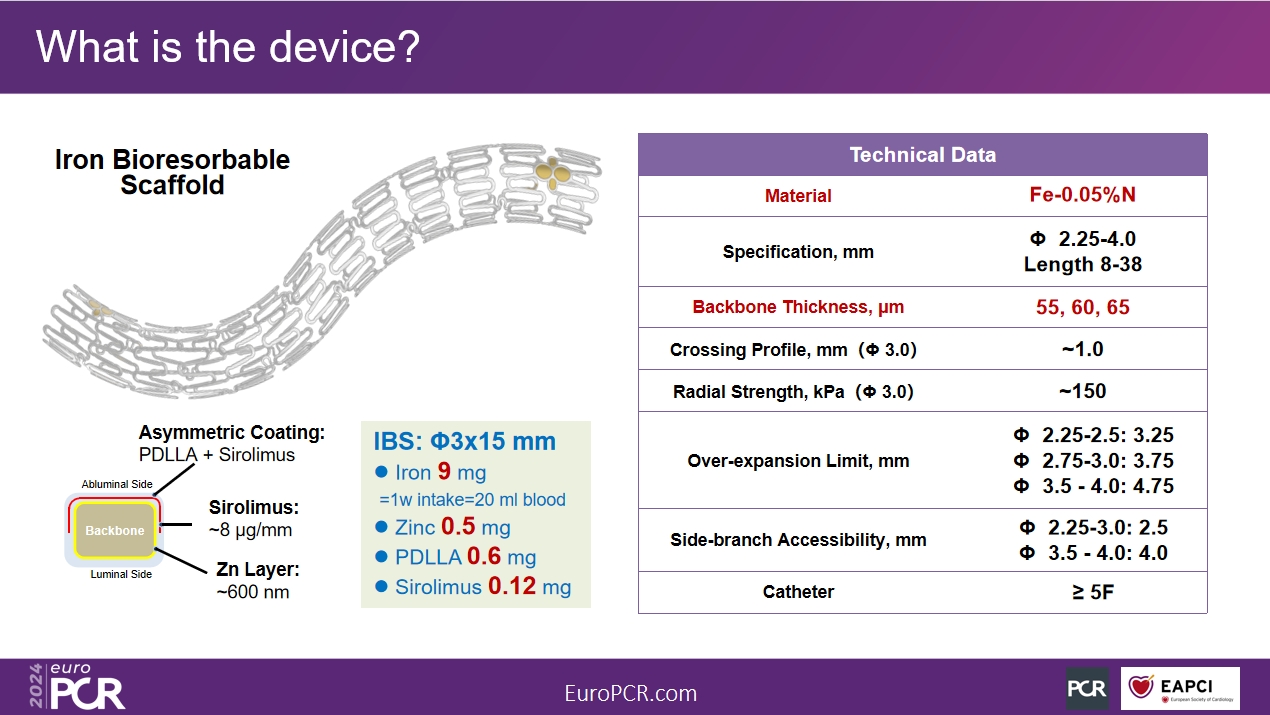
Figure 1. IBS™ Coronary Scaffold
Eighteen years of dedicated research and development
The world's first fully biodegradable iron-based bioresorbable coronary scaffold
Coronary heart disease is one of the most common cardiovascular diseases, with high morbidity and mortality, which seriously threatens human health. Percutaneous coronary intervention has developed rapidly due to its advantages of being minimally invasive, time-saving, safe, and efficient, and has now become the mainstream treatment for coronary heart disease. According to Frost & Sullivan's forecast, the global use of coronary intervention stents is expected to exceed 12 million by 2030, and its market size is expected to grow to US$9.1 billion, making it the medical device with the largest single market capacity. However, permanent metal coronary stents are non-degradable and will accompany patients throughout their lives after being implanted in the human body, requiring patients to take medication for life, bear the risk of long-term stent fatigue fracture, and have a series of problems such as limited secondary intervention for vascular restenosis and atherosclerosis. In recent years, with the continuous development of medicine, "intervention without implantation" revascularization has become a development trend in the field.
IBS™ Coronary Scaffold was independently developed by LifeTech over the past 18 years and is the world's first fully biodegradable iron-based bioresorbable coronary scaffold. The backbone is made of high-strength and high-plasticity high-purity nitrided iron tubes. The stent wall is thin (55~65μm) and has strong support. Innovative material research and unique technical paths have enabled IBS™ Coronary Scaffolds to retain the advantages of permanent metal coronary stents, such as complete specifications (φ2.25~4.0*8~38mm), superior physical properties, good biocompatibility, and simple operation (no PSP and slow expansion). It also has the characteristics of being fully absorbable, which can effectively avoid a series of long-term prognostic problems that may be caused by implanting permanent metal stents.
Leading the world with promising future
The IBS™ coronary scaffold started its pre-market clinical study in China (Phase III) in March 2018, with Academician Runlin Gao of the Fuwai Hospital of the Chinese Academy of Medical Sciences as the principal investigator (PI), and Academicians Ge Junbo and Han Yaling, as well as nearly 40 clinical research centers and experts across the country, were invited to participate.
In April 2023, the three-year follow-up results of the FIM (Phase I clinical study) of the IBS™ Coronary Scaffold were published online in the international authoritative medical journal EuroIntervention. The data showed that the target lesion failure rate (TLF) of the IBS™ Coronary Scaffold was only 2.2% six months after implantation, and the TLF was stable at 6.7% one, two, and three years after implantation. No death, myocardial infarction, or thrombosis occurred during the entire follow-up period; the target vessel neointimal coverage rate of the IBS™ Coronary Scaffold was as high as 99.8% six months after implantation, and reached 100% after one year; there was no acquired poor adhesion during the entire degradation process, and the degradation was completed two to three years after successful implantation. It has been preliminarily proved that the IBS™ Coronary Scaffold has good mid-term safety and effectiveness in simple primary coronary lesions. In addition, the vascular lumen area of the IBS™ Coronary Scaffold continued to expand six months after implantation, which is exactly the expected development trend of absorbable stents and reflects the unique clinical advantages of the IBS™ Coronary Scaffold. The product has now completed the five-year FIM follow-up with positive results.
The IBS™ Coronary Scaffold has been published, further strengthening the evidence-based medicine of this world-first product and the confidence in its future successful commercialization. At the same time, the product is undergoing a one-year follow-up of a single-group target value study (i.e., "Phase III clinical study") in China, and the progress is smooth. The fully degradable metal coronary stent made of iron has demonstrated great clinical application potential and bright prospects in current clinical studies. In addition, the IBS™ Coronary Scaffold has successfully submitted an application for EU CE registration, and it is expected to become the world's second successfully commercialized iron-based bioresorbable scaffold product after the IBS Angel™ Iron Bioresorbable Scaffold System.
With the continuous improvement of subsequent clinical research and evidence-based medicine, the access of this revolutionary innovative product to the global market will be further promoted, bringing unprecedented, safe, and effective treatment methods to coronary heart disease patients around the world in the near future, and will actively promote the treatment of related diseases to enter the iron-based absorbable era!
