For EU Regions Only
Product features
1. Bioresorbable scaffold with minimum profile diameter
The scaffold is compatible with 4F sheath and is applicable to fine infant blood vessels of newborn.
2. High post-expansion limit
The post-expansion limit at the time of implantation is nominal diameter +0.75mm.
3. Reasonable degradation profile
Effective support for 6 months and complete degradation in about 2 years, without restricting blood vessels.
Technical Data
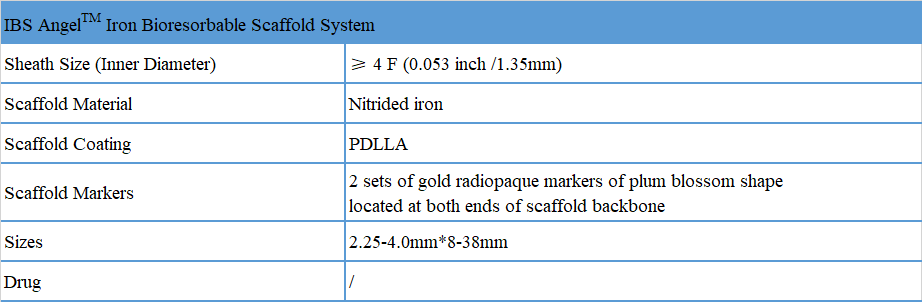
Product Specification
Size specification table
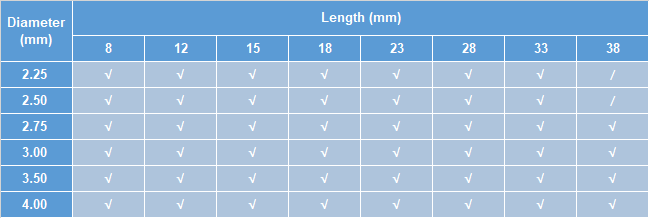
Note: “√ ” indicates that the sizes of the scaffold are available;
“/ ” indicates that the sizes of the scaffold are not available.
541-EN-01 V1.0
产品特点
1. 最小轮廓外径的可吸收支架
4~7F鞘管兼容,可适用于新生儿细幼血管。
2. 高后扩极限
植入0时刻的后扩极限为名义直径+1mm
3. 可二次介入扩张
支架支撑杆上预设多个通孔的断点设计,保证6个月后尽早周向解构,有利于必要时的二次介入扩张,不束缚血管近期长大。
4. 合理的降解曲线
6个月有效支撑,~2年全降解,不束缚血管远期长大
产品规格
铁基可吸收支架系统尺寸规格表

注:“√”表示有该尺寸规格的支架;“/”表示没有该尺寸规格的支架