Product Features
1. Thinnest
The world's thinnest scaffold with 70 μm , the world's only iron-based bioresorbable technology path.
2. Longest
The breakthrough 118mm length, can cover the lesion length up to 200mm by overlapping use which can meet the real world clinical application needs.
3. Excellent operation performance
The scaffold is compatible with 5F catheter and has excellent visibility. The nominal diameter of the post expansion limit is +0.75mm.
4. High drug utilization
The content of sirolimus is 1.4μg/mm2, with most of the drug being located on abluminal surface of the struts.
5. Reasonable degradation profile
Effective support for 6 months and fully degradable in about 1.5 years, allowing secondary intervention expansion when necessary.
Technical Data
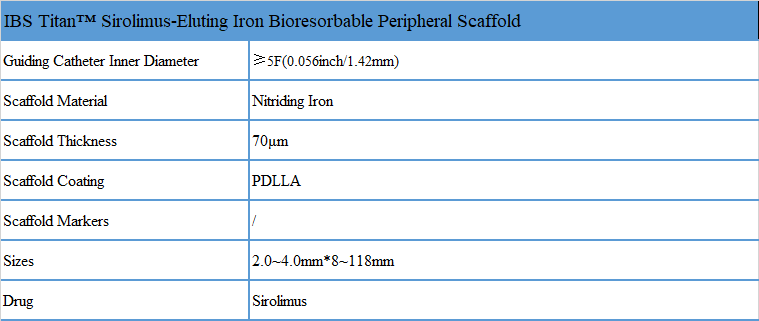
Product Specification
Size Specification Table
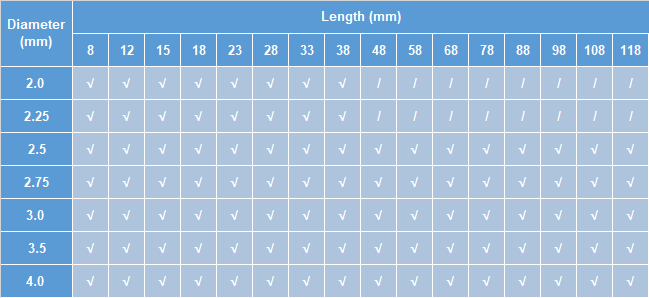
Note:“√”indicates that there is a scaffold of this size;“/”indicates that there is any scaffold of this size.
产品特点
1. 最薄
全球最薄70微米支架杆,全球唯一铁基可吸收技术路径。
2. 最长
突破性118mm长度的支架,重叠使用可覆盖病变长度达到200mm,满足真实世界临床应用需求。
3. 易操控
5F导管兼容,支架本体可视性优异,后扩极限为名义直径+0.75mm。
4. 高药物利用率
雷帕霉素含量,1.4μg/mm2,药物富集在支架杆离腔侧
5. 合理的降解曲线
6个月有效支撑,~1.5年全降解,允许必要时的二次介入扩张。
产品规格
可吸收药物洗脱外周支架尺寸规格表
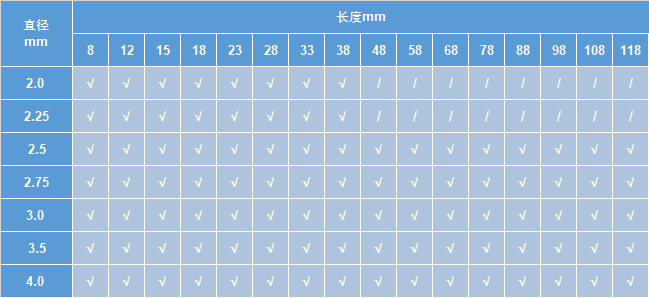
注:“√”表示有该尺寸规格的支架;“/”表示没有该尺寸规格的支架