From September 21st to 25th, 2018, the 30th American Transcatheter Cardiovascular Therapeutics (TCT 2018) was held in San Diego, USA. As the pride of the industry's leading innovative cardiovascular minimally invasive interventional medical device company and national brand, Biotyx Medical (Shenzhen) Co., Ltd. has brought IBS®™ sirolimus-eluting iron bioresorbable coronary scaffold, developed over 12 years, to participate in this world advanced meeting in the field of cardiovascular intervention.
On the 21st local time, the preliminary results of the world’s first fully degradable iron-based scaffold IBS-FIM study were officially released for the first time, confirming its preliminary safety and effectiveness in human applications. The fully degradable metal coronary scaffold using iron as a material shows its great potential and bright prospects.

Research Bcckground of IBS-FIM
The Biotyx’s IBS™ sirolimus-eluting iron absorbable coronary scaffold system is the only fully degradable scaffold and the only in the world that uses iron as a material, and is currently the only fully degradable scaffold with a thin wall design. The wall thickness of the scaffold main body is only 50μm-55μm, which is thinner than the mainstream permanent drug scaffold, but its strength is greater than that of the cobalt-chromium alloy. The thin-walled design is conducive to rapid intimalization after stent implantation, thereby reducing the risk of postoperative thrombosis, and preventing scaffold thrombosis more unique and efficiently. The slow-release layer covered on the surface of the scaffold body can delay the degradation of the scaffold body and ensure that the support strength does not decrease in the early stage of stent implantation. The outermost drug-carrying coating retains the drug only on the side that contacts with the blood vessel, which increases the drug utilization efficiency, reduces the total drug amount, and reduces the incidence of restenosis. The degradation of the drug-loaded coating can also promote the degradation of the scaffold body, and neutralize the degradation products of polylactic acid, reducing the inflammatory reaction caused by polylactic acid.
The degradation elements of the IBS®TM scaffold are necessary iron and zinc for the human body, and the amount of iron and zinc released by the scaffold every day is much smaller than the human intake. It has a range of scaffold specifications, indications, and clinical operations comparable to permanent stents under the condition of similar drug coating laying and full degradation characteristics.
At the TCT, Professor Kefei Dou announced the preliminary results of the IBS-FIM study for the first time. The study plans to enroll 45 patients and complete a 3-year imaging follow-up and 5-year clinical follow-up. The primary endpoint was 6 months of target lesion failure (TLF) and 6 months of late lumen loss (LL).

(The TCT Congress first reported the progress of IBS-FIM research)
So far, Fuwai Hospital has enrolled 20 patients with coronary heart disease who meet the plan to implant IBS®TM scaffolds, and 17 of them have completed 1 month of clinical follow-up. The average age of all subjects was 52.8 ± 1.99 year old, the average length of the lesion was 16.4 ± 0.49mm, the average reference vessel diameter was 3.3 ± 0.56%, the implantation success rate was 100%, and no intraoperative complications occurred.
From the 1-month follow-up results, the target lesion failure (including cardiogenic death, TV-MI and CT-TLR) is 0, and the patient’s clinical composite end point (including all-cause death, all myocardial infarction, and any cause Revascularization again) is 0. There were no serious adverse events related to thrombosis, instruments or surgery.
Professor Kefei Dou also mentioned that at the opening ceremony of the CIT conference on March 23rd this year, the world's first implantation of IBS scaffolds was broadcast live. The 6-month postoperative imaging and OCT follow-up were completed on September 18th. Six months of coronary angiography showed that the lumen remained unobstructed, and OCT showed no signs of poor scaffold attachment, and the intima coverage reached 100%, with an average intimal thickness of about 0.1 mm, and signs of stent degradation could be seen.
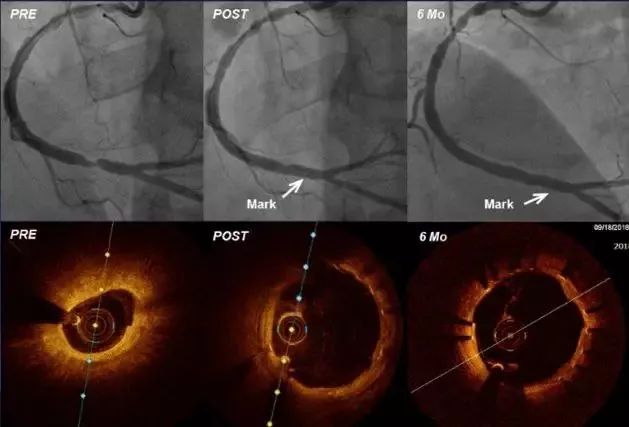
(Result image of OCT)
The above results indicate that the safety and effectiveness of the IBS®TM scaffold in the human body has been initially confirmed. Similar to the permanent scaffold, the scaffold performance and operation method ensure the 100% successful implantation rate of the IBS®TM scaffold. The total wall thickness of the 70μm scaffold and the fast endothelialization rate ensure that the endothelium can be completed before the scaffold begins to degrade , which can effectively reduce the risk of thrombosis. The fully degradable metal coronary scaffold made of iron shows its great potential and bright prospects.
Expert Reviews
Academician Runlin Gao stated:
The IBS®TM scaffold is the only biodegradable scaffold made of iron in the world. The results of animal experiments show that after scaffold implantation, the vascular endothelium heals quickly, the rate of restenosis is low, and there is no obvious inflammatory reaction. Since the endothelial crawling began earlier than mainstream permanent metal scaffolds, there was no case of broken rods extending into the lumen after degradation, and no thrombus was seen. The preliminary results of this study show that the implantation of IBS®TM scaffolds in the human body is also endothelializing quickly. The degradation of the scaffold has been seen in 6 months, but there is no safety incident in the patency of the lumen, which is really encouraging!
Professor Bo Xu said:
As the world's first Chinese original technology absorbable iron-based scaffold, IBS®TM scaffold not only shows its superior performance, good clinical results, but also finds many new phenomena from the academic perspective. For example, the volume expansion phenomenon found in the six-month OCT follow-up results has never been seen in the development history of absorbable polymer scaffolds or traditional permanent metal scaffolds. It has brought the academic world new enlightenment and exploration directions, and its unique characteristics and new phenomena will surely attract the attention of the world.
Biotyx Medical (Shenzhen) Co., Ltd. stands on the top of the world in the field of cardiovascular intervention. It once again successfully demonstrated the excellent product design and safety and effectiveness of IBS®TM sirolimus-eluting iron bioresorbable coronary scaffold, and showed the company's independent innovation strength. With the accumulation of surgical experience and related data, it will further prove the safety and effectiveness of IBS®TM sirolimus-eluting iron bioresorbable coronary scaffold. We look forward that this excellent and innovative product can serve patients worldwide and allow more patients to enjoy a healthy "heart" life in the near future!
