From June 26th to 29th, 2019, the Congenital, Structural and Valvular Heart Disease Interventional Treatment Conference (CSI Frankfurt 2019) was grandly held in Frankfurt, Germany. The event will have an in-depth discussion on the main topics such as catheter interventional treatment of congenital, structural and valvular heart disease, aiming to support and promote global multidisciplinary collaborate by providing training programs and educational opportunities for medical professionals from all over the world, which will advance the research and treatment of structural, valvular and congenital heart diseases worldwide.

At the CSI Frankfurt 2019, the world premiere implantation surgery of the IBS® Angel ™ iron bioabsorbable scaffold, independently developed by Biotyx, became the academic highlight of the audience. On June 27th, at the prime time of the much-anticipated main venue, Professor Mazeni Alwi, a well-known expert in the field of global pediatric congenital from the National Heart Institute of Malaysia, served as the main surgeon and showed his PDA stenting by using IBS® Angel™ iron-base scaffold to the global audience, which is epoch-making significance. The child underwent surgery was just 12 days old and had congenital tricuspid atresia. The arterial catheter was connected to the descending aorta. Professor Mazeni Alwi chose the 15mm long and 4mm diameter IBS® Angel™ iron bioabsorbable scaffold. It provides survival time for further elective cardiac surgery for children. The entire operation took a short time and the process was very smooth. A warm applause broke out at the conference site. Experts at the meeting praised the wonderful operation, making very lively interaction.
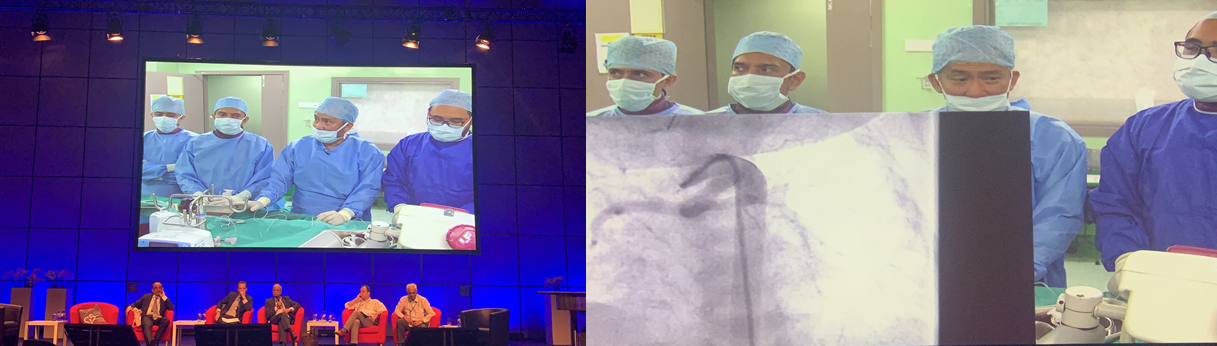
(World premiere of IBS Angel ™ iron bioabsorbable scaffold implantation surgery)
Professor Mazeni Alwi also announced the clinical progress of the IBS® Angel™ iron bioabsorbable scaffold at the National Heart Institute of Malaysia. The center is currently the world’s first human clinical trial center for the IBS® Angel™ scaffold. The main purpose of this study was to evaluate the feasibility, safety, and effectiveness of the IBS® Angel™ scaffold in simple arterial catheters. This will provide a basis for future large-scale research in more complex arterial catheters. In this trial, the IBS® Angel™ scaffold was implanted in a simple arterial catheter (a quite short and straight arterial catheter connected to the aortic arch / descending aorta). The patient is a neonate with an arterial catheter-dependent complex congenital heart disease, such as complete ventricular septal pulmonary atresia (PAIVS). The study observed the situation of the IBS® Angel™ scaffold from implantation to discharge and followed up 9 months after discharge. The end event was death within 9 months after implantation, and unintended reintervention or reoperation. The current research results show that all the operations were successful and the preliminary results are positive. The ease of use of the IBS® Angel™ scaffold is comparable to the bare cobalt-chromium alloy scaffold; follow-up results show that the implanted children recover well, and some children have successfully received follow-up cardiac surgery to regain health.
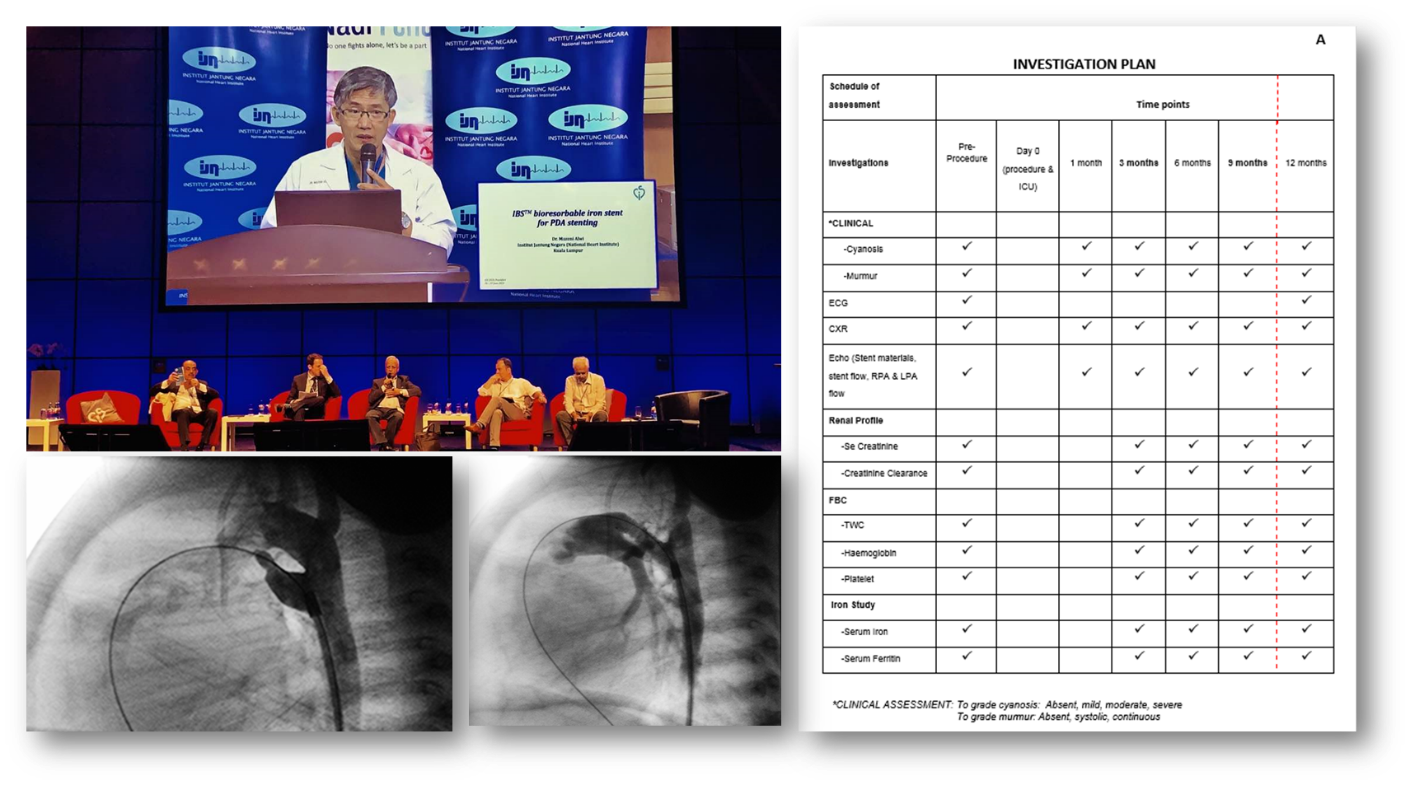
(Professor Mazeni Alwi announced the clinical progress of IBS Angel ™ iron bioabsorbable scaffold)
Professor Mazeni Alwi said in his speech: PDA scaffold can temporarily relieve the symptoms of arterial catheter-dependent complex congenital heart disease, giving children with complex heart disease time and opportunity to wait for heart surgery. While IBS® Angel™ iron bioabsorbable scaffold has more advantages. It is very suitable for children with this kind of congenital heart disease. Its small size can be suitable for newborns and infants. And it is conducive to ligation of arterial catheter or placement of occlude, and reduce complications after cardiac surgery. At present, when the existing metal bare scaffold is implanted, it needs to be taken out by surgical means while performing the repair operation again, which is company with open chest trauma and high postoperative complications. The absorbable scaffold does not damage the blood vessel of the child and is easier to receive further surgical treatment at a later stage. Through the clinical trial of IBS® Angel™ iron bioabsorbable scaffold applied to children with arterial catheter-dependent complex congenital heart disease, combining with the staged clinical follow-up records, it is initially shown that IBS® Angel™ iron bioabsorbable scaffold is feasible, safe and effective in a simple arterial catheter. This has laid the foundation for more large-scale research in more complicated arterial catheters in the future. Studies have shown that the scaffold can be further applied to many congenital heart diseases such as pulmonary artery stenosis, right ventricular outflow tract stenosis, and pulmonary vein stenosis.

The IBS®™ iron bioabsorbable scaffold series is the “intellectually made” of Lifetech for 12 years. The IBS® Angel™ iron bioabsorbable scaffold is a product that develops on the basis of IBS®™ coronary scaffold and optimizes for pulmonary vascular indications. Professor Mazeni Alwi successfully demonstrated the excellent product design and excellent handling performance of the IBS® Angel™ iron bioabsorbable scaffold. Experts in the cardiovascular field presented all highly praised IBS® Angel™. LifeTech's IBS®™ iron bioabsorbable scaffold is the world's only fully degradable vascular scaffold using iron-based materials, and is currently the only fully degradable scaffold with a thin-wall design. The wall thickness of the metal rod of the scaffold is only about 55 μm, which is thinner than the mainstream permanent drug scaffold, but the overall mechanical properties of the scaffold are not inferior to the cobalt-chromium alloy scaffold. The thin-walled design is conducive to rapid intimalization after scaffold implantation, thereby reducing the risk of postoperative thrombosis, and preventing scaffold thrombosis more uniquely and efficiently. The degradation products of IBS®™ iron bioabsorbable scaffolds are the essential trace elements iron and zinc, and the amount of iron and zinc released by the scaffolds per day is much less than the average daily intake of the human body.
At this influential structural heart disease intervention therapy conference worldwide, Biotyx once again demonstrated the company's strong independent innovation strength. The IBS® Angel™ iron bioabsorbable scaffold has brought new directions of inspiration and exploration to the treatment of congenital heart disease in children around the world. Its unique characteristics have attracted worldwide attention. With the accumulation of doctors' surgical experience and relevant clinical data, we look forward to the excellent innovative products serving children with congenital heart disease in the near future, allowing more children to get rid of the disease as soon as possible and enjoy healthy life brought by technology.
