
Previous:First Implantation of IBS Angel™ Completed in Argentina
Next:Biotyx's IBS Angel™Iron Bioresorbable Scaffold System obtains EU CE MDR certification
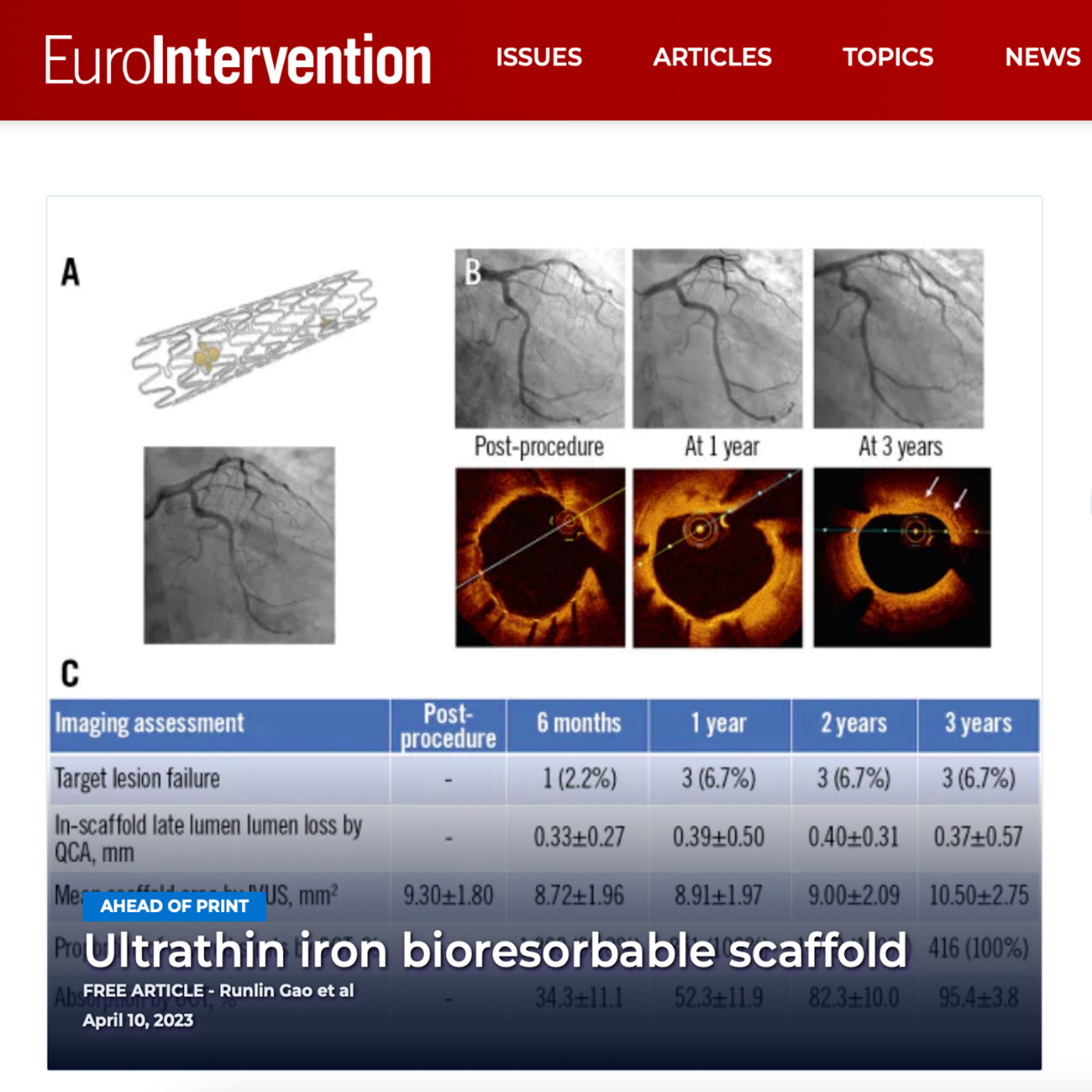 The world's first product independently developed by LifeTech Scientific Holdings' subsidiary Biotyx Medical (Shenzhen) Co., Ltd., the IBS™ Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold System (hereinafter referred to as: IBS™ Coronary Scaffold), has successfully completed the three-year follow-up of the feasibility (FIM) clinical study. On April 10, 2023, the results of the study were published online by the team of Academician Gao Runlin of the Fuwai Hospital of the Chinese Academy of Medical Sciences in the international authoritative medical journal EuroIntervention. This not only represents the high attention paid by the international industry to this study and the unanimous affirmation of the innovation of this product, but also another perfect appearance of China's medical innovation technology in the global academic highland.
The world's first product independently developed by LifeTech Scientific Holdings' subsidiary Biotyx Medical (Shenzhen) Co., Ltd., the IBS™ Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold System (hereinafter referred to as: IBS™ Coronary Scaffold), has successfully completed the three-year follow-up of the feasibility (FIM) clinical study. On April 10, 2023, the results of the study were published online by the team of Academician Gao Runlin of the Fuwai Hospital of the Chinese Academy of Medical Sciences in the international authoritative medical journal EuroIntervention. This not only represents the high attention paid by the international industry to this study and the unanimous affirmation of the innovation of this product, but also another perfect appearance of China's medical innovation technology in the global academic highland.
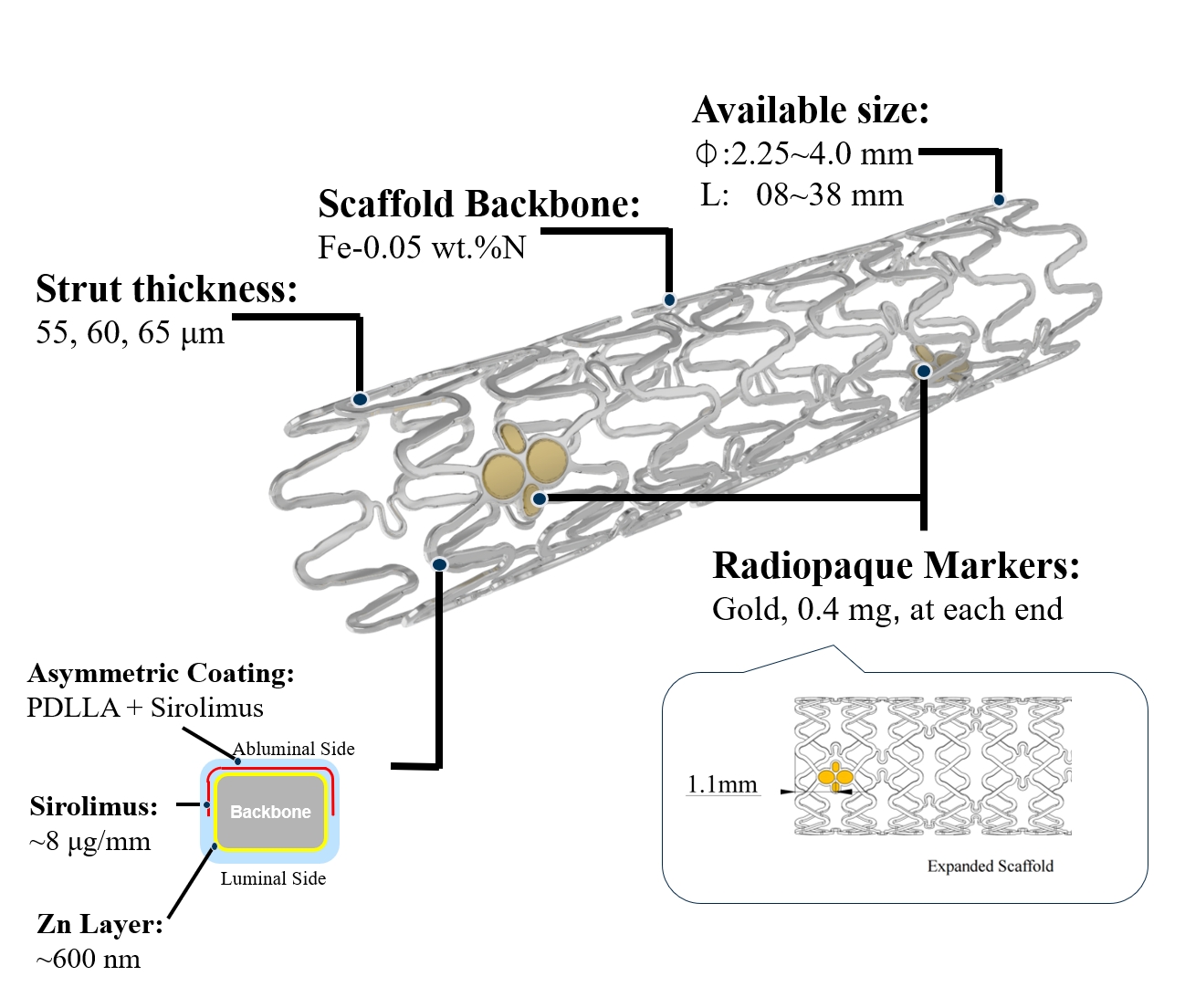
Figure 1. IBS™ Coronary Scaffold
Leading the world with positive data
The FIM clinical study of IBS™ Coronary Scaffold began in 2018 at the Fuwai Hospital of the Chinese Academy of Medical Sciences, with Academician Gao Runlin as the principal investigator (PI). The subjects were randomly assigned to two cohorts in this clinical study, of which cohort 1 has completed six months and two years of imaging follow-up, and cohort 2 has completed one year and three years of imaging follow-up, with preliminary results positive. The completion of the three-year FIM clinical follow-up marks that the commercialization process of this innovative product is continuing to advance steadily.
The operation method of IBS™ Coronary Scaffold is the same as that of metal permanent stent, without forced PSP and slow expansion. All stents in clinical studies were successfully implanted in patients without surgical complications, and the device, lesion and clinical success rates were 100%. Clinical data showed that the target lesion failure rate (TLF) was only 2.2% six months after stent implantation, and the TLF was stable at 6.7% one, two and three years after implantation, and no death, myocardial infarction or thrombosis occurred during the entire follow-up period. At the same time, OCT analysis results showed that the neointimal coverage rate was as high as 99.8% six months after stent implantation, and reached 100% after one year. It preliminarily shows that IBS™ Coronary Scaffold has good mid-term safety and effectiveness in simple primary coronary lesions.
At the same time, there were no stenotic changes in the lumen during the three-year follow-up period, and no long-term acquired malapposition occurred during the degradation process of the stent. Intra-segmental late lumen loss (LLL) was 0.25 ± 0.26 mm at six months, 0.27 ± 0.45 mm at one year, 0.27 ± 0.35 mm at two years, and 0.21 ± 0.38 mm at three years. Unlike other stents whose lumen area continues to decrease significantly as the stent implantation time increases, the lumen area of the IBS™ Coronary Scaffold continues to expand six months after implantation, steadily increasing from 7.22mm2 to three months after implantation. 8.03mm2 per year. This is the expected development trend of absorbable stents and also reflects the unique clinical advantages of IBS™ Coronary Scaffold.
From the FIM clinical study, it was observed that the absorption rate of the IBS™ Coronary Scaffold reached 82% ± 10% two years after implantation and 95% ± 4% three years after implantation, fully proving that the iron-based stent can be used in the human body. Be safely absorbed. Fully degradable metal coronary stents made of iron show great application potential and bright prospects.
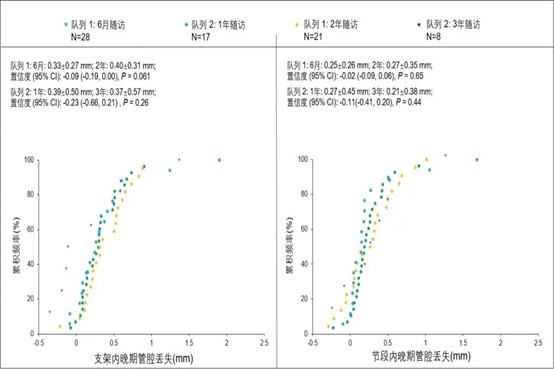
Figure 2. In-stent and intra-segmental late lumen loss (LLL)
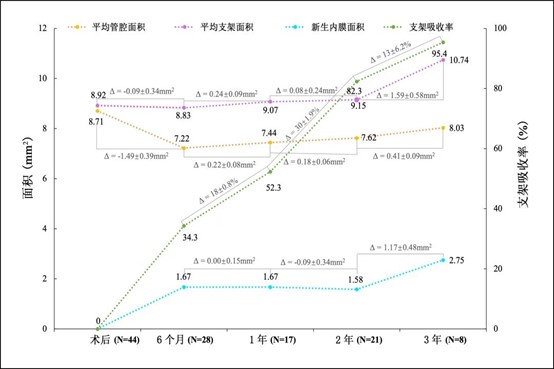
Figure 3. OCT follow-up results
Craftsmanship builds dreams and leads the future
IBS™ Coronary Scaffold is the world's first fully degradable iron-based absorbable coronary stent. Its matrix is made of high-strength and high-plasticity high-purity nitrided iron tubes. The stent wall is thin (the total wall thickness of the stent rod is only 70μm) and has strong support. Innovative material research and unique technical paths have enabled IBS™ Coronary Scaffolds to retain the advantages of permanent metal coronary stents, such as complete specifications, superior physical properties, good biocompatibility, and simple operation. It also has the characteristics of being fully absorbable, which can effectively avoid a series of long-term prognosis problems that may be caused by implanting permanent metal stents.
The publication of the three-year follow-up results of the FIM clinical study of the IBS™ Coronary Scaffold further strengthens the evidence-based medicine of this innovative product, and will also lay a solid foundation for the global development of this product and other core products on the iron-based bioresorbable material platform of Lifetech Scientific. The IBS™ Coronary Scaffold has completed the enrollment of all subjects in the Phase II randomized controlled clinical study in December 2022, and its prospective, multi-center, single-group target value study (i.e., Phase III clinical study) is currently enrolling clinical subjects.
LifeTech's Iron-based bioresorbable material platform has experienced sixteen years of steady development and is an innovator and leader in the field of global bioresorbable stents. In the finals of the 2022 National Disruptive Technology Innovation Competition, which ended earlier, it entered the finals after layers of screening and competition among more than 2,800 technical projects, and finally stood out from 157 high-quality projects with disruptive potential, and won the highest award of the finals - the Excellence Award, with a unanimous vote, and entered the Ministry of Science and Technology's disruptive technology candidate pool. It is believed that with the continuous improvement of subsequent clinical and evidence-based medicine, this revolutionary technology will bring unprecedented, safe and effective treatment methods to patients around the world, and will actively promote the treatment of related diseases into the iron-based bioresorbable era!
